From an agricultural standpoint pH is important because it strongly affects plant growth, nutrient availability,elemental toxicity and microbial activity. In an agricultural sense, soil pH indirectly affects plant growth.
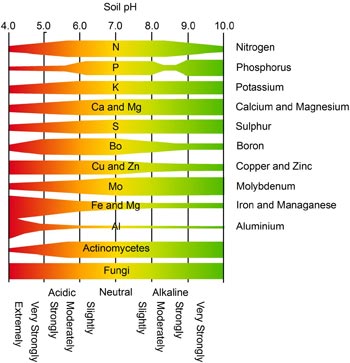
This is because various mineral nutrients are readily available in varying concentrations depending on the pH of soil. At certain pH levels, certain mineral nutrients remain with other minerals and are unavailable to the plant.
For example and with respect to cotton, soil pH should be in the range of 5.5 – 7.0. If the pH is greater than 7, the availability of some nutrients such as zinc may become limiting. This may be the case in the arid and semi-arid cotton growing areas where the soil is moderately- (i.e. pH 7-8.5) to strongly-alkaline (i.e. > 8.5).
Conversely, if pH is less than 5, the availability of some nutrients such as phosporus, calcium, magnesium and molybdenum is very low and so plant uptake is limited.
In addition, some generally insoluble cations (e.g. iron and aluminium) may be released into the soil solution. The result will be reduced plant vigour owing to the sensitivity of many plant roots to aluminium toxicity.
Adjustment of soil pH will often result in the re-adsorption or release of the nutrient back into soil solution. It is therefore argued that pH is the single most important diagnostic chemical measurement of soil.The term pH is short for potential hydrogen. This is because pH indicates the concentration of H+ activity in the soil solution. It is expressed as follows:
pH = -log (H+)
Soil Reaction or pH describes the acidity or alkalinity of a soil. The pH scale ranges from 0 -14. Values between 0 and 7 are said to be acidic with a pH value of 1.0 being very acid and a pH value of 6.0 said to be slightly acid.
Values between 7 and 14 are said to be basic or alkaline; whereby a larger number indicates stronger alkalinity. The value of 7 is the midpoint of the scale and is neutral. The pH of pure water is neutral.
Because the pH scale is logarithmic, going down the scale from a pH value of 7, each number is ten times (x10) more acid than the one before. For example,soil pH of 6 is x10 more acid than neutral (i.e. pH 7). Further, a soil with a pH of 5 is x100 more acid than neutral (pH 7). In other words the more hydrogen ions there are the more acid is the soil.
The range of soil pH (i.e. 95 % of most soil) is generally between 4 and 10. The distribution of acid and alkaline soil is in general a function of climate.
Acidic soil is most common where rainfall is high and free drainage favors leaching and biological production of acid. This is because most of the exchangeable cations of calcium, magnesium, potassium and sodium are leached. This process occurs because of the introduction of a weak (i.e. carbonic acid) into a soil profile in one of two ways.
In the atmosphere, as raindrops form and fall through the air, CO2 dissolves in the rainwater to form carbonic acid:
H2O (aq) + CO2 (g) ↔ H2CO3 (aq)
This weak acid is harmless to plants and animals, but over a prolonged period of time it is able to dissolve rocks, like feldspar and limestone. This is because in soil solution and at pH values above 6, the carbonic acid quickly breaks down to liberate H+ ions as follows:
H2CO3 ↔ H+ (aq) + HCO3- (aq)
Now there is an excess of H+ so this dilute solution is acidic with additional release of H+ occurring as follows:
HCO3- (aq) ↔ H+ (aq) + CO32- (aq)
This is the reason why in equilibrium with the atmosphere water has a slightly acidic pH of 5.6. This is also the reason why naturally acidic soil types are found in high rainfall areas.
Carbonic acid is a weak acid is only partially dissociated to release H+, whilst a strong acid is almost completely dissociated. Carbonic acid is too weak to dissociate much at pH < 5 but has significant acidifying effect in alkaline (pH > 7) and neutral soil where it can release plant nutrients and promote mineral weathering.
In addition and because soil organic matter (OM) production is generally greater in high rainfall areas, carbonic acid is catalysed by micro-organisms.
Here bacteria mediate oxidation of decaying OM to CO2. This oxidation reaction can be considered by representing OM by a simple carbohydrate (CH2O). The overall reaction is as follows:
CH2O (s) + O2 (g) → H2O (aq) + CO2 (g) ↔ 2H+ (aq) + CO32- (aq)
Soil processes that push these reactions to the right include root respiration and decomposition of soil OM by microbes. In both cases high levels of CO2 are produced.
As a consequence, the percolating water is slightly acidic and this gradually results in the acidification of soil. This is because the percolating water replaces and leaches soluble exchangeable cations (i.e. calcium, magnesium, potassium and sodium) out of the soil profile. The exchangeale cations have been replaced with hydrogen ions (i.e. H+).
Conversely, alkaline soil (e.g. Calcarosol, Vertosol and Sodosol) is synonymous with arid and semi-arid landscapes where evapotranspiration exceeds rainfall, which favors retention and accumulation of exchangeable cations.
In these climatic regions the alkaline nature of the soil is a function of the calcium carbonate present in subsoil horizons.
The carbonate accumulates from carbonate rich dust (CaCO3) which is initially blown onto the soil surface. As described above water in precipitation (H2O) combines with atmospheric and soil carbon dioxide (CO2) to make weak carbonic acid (H2CO3).
Calcium carbonate in and at the soil surface reacts with the carbonic acid. This dissolves CaCO3 releasing Ca2+ (aq) making it mobile with both ions translocated deeper into the soil profile with percolating water:
H2CO3 + CaCO3 (s) ↔ Ca2+ (aq) + 2HCO3- (aq)
Dry conditions at depth lead to precipitation of secondary carbonates:
Ca2+ + 2HCO3- + H2O ↔ CO2 + CaCO3 (s)
High pH therefore indicates the soil is fully saturated with exchangeable cations and free CaCO3 is present in the soil. Soil profiles high in carbonate have pH values of approximately 8.3.
Carbonates (e.g. CaCO3) may be in the form of concretions or diffuse areas. Effervescence with hydrochloric acid (HCl) suggests the presence of carbonates.
In order to measure pH, laboratory methods require the use of a glass electrode pH meter in a soil/liquid system of varying proportions. The liquid is either distilled water or a range of salts (e.g. 0.01 M Calcium Chloride - CaCl2) with the soil:water ratio varying from 1:1 (i.e. pH1:1) to 1:5 (i.e. pH1:5).
A CaCl2 solution is preferred (i.e. pHCaCl2) as water excessively dilutes the soil solution and therefore leads to an overestimation of pH by about 0.5 for most soil types. In Australia, however, soil pH1:5 is usually determined. All data shown in terraGIS has been determined using the pH1:5 method.
Regardless of the method used, the extract is measured using a pH meter, which displays the electrical potential difference between the glass electrode containing a solution of a known pH and the test solution.
In order to determine soil pH1:5, using the electrometric method, the following steps are undertaken:
- Place 5 grams of soil into a pop-top tube;
- Add 25 ml of distilled water;
- Place the sealed pop-top tube onto spinning wheel;
- Remove pop-top tube after 20 minutes; and,
- Measure and record soil using a pH meter.
Soil |
Unit |
Very low |
Low |
Moderate |
High |
Very High |
pH |
|
<4.5 |
4.5-6.0 |
6.0-7.0 |
7.0-8.3 |
>8.3 |
When soil pH is very low to moderate, lime is commonly added since it will dissolve to form acid-neutralizing constituents (i.e. Ca2+) and also provide a source of Ca.
However, in neutral and alkaline soils, lime is very stable and will not rapidly dissolve. Adding lime in these conditions will do very little to improve nutrient availability and may even further reduce the solubility of phosphorus (P) and some micronutrients.