Soil as a complex system supports the growth of plants. Its ability to do this is not only dependent on the adequate supply of nutrients, but also upon the adequate supply of water and air. This requires sufficient pore spaces to allow plant (root) growth but at the same time provide substantial water for storage and aeration for plant respiration.
In general, water can cause soil mineral particles to swell and shrink and adhere to each other. In this way it can initiate the process of structural aggregation. It is also intimately involved in numerous chemical reactions that weather primary and secondary clay minerals (e.g. hydrolysis), releasing chemical nutrients into the soil solution and create acidity.
In soil, water is stored in pores and on organic and inorganic soil mineral particle surfaces that arise from the physical aggregation of soil particles (i.e. clay, silt and sand). In this way substantial volumes of water can be held in soil. It is estimated that to a depth of 1 m, soil at field capacity can hold approximately 800,000 L (0.8 ML) of water.
However, soil water storage is never permanent, exhibiting substantial variation in space and time. This is particularly the case near the soil surface where under natural systems water is added through precipitation and via irrigation in man made systems. At the same time as water enters soil it can be removed rapidly in the presence of active roots. In this regard water is removed in gaseous form (i.e. transpiration and evaporation). Where it is not utilized by plants, water can be removed in liquid form (e.g. deep drainage to groundwater).
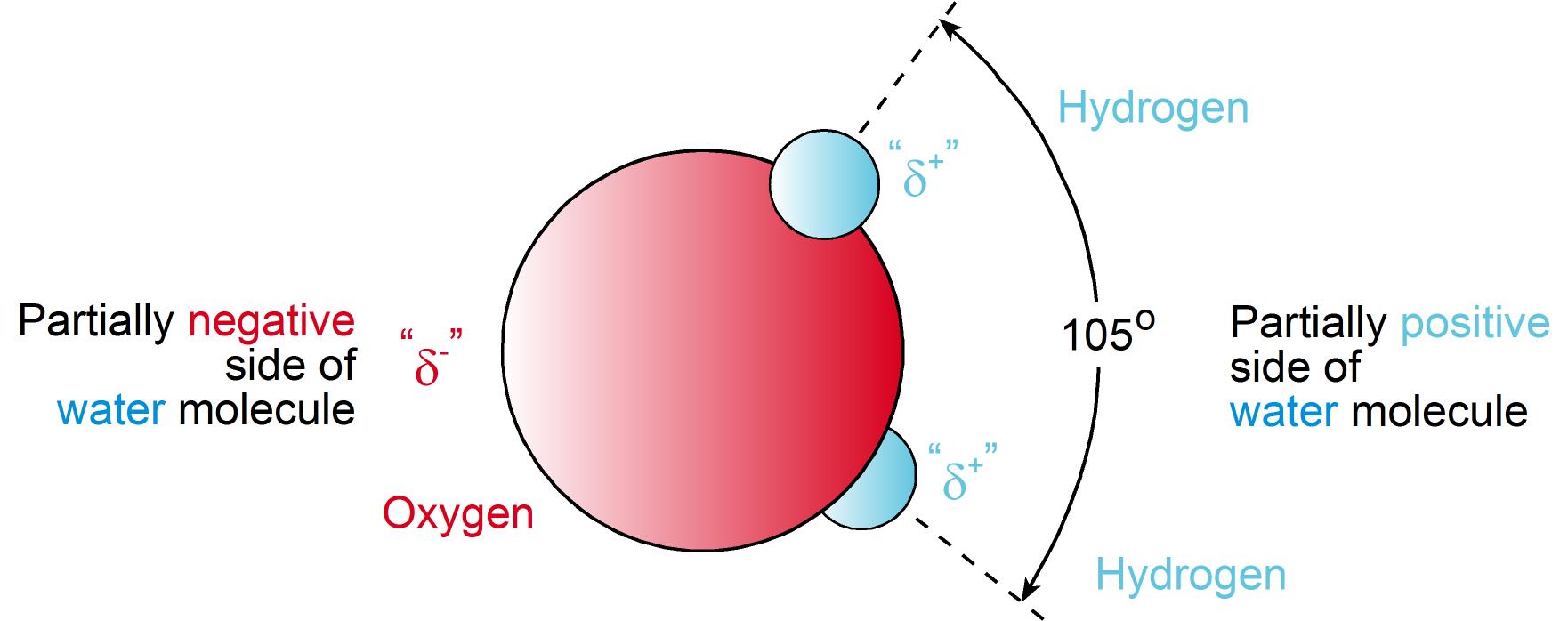
To understand how water is actually held in soil and is made available to plants it is important to understand how it binds and is released. The first thing to realise is that an individual water (H2O) molecule is neutral. That is, the negative charge associated with the oxygen atom (i.e. -2) is equal to the number of positive charges associated with the two individual hydrogen atoms (i.e. +1 each).
However, the overall charge is asymmetrical. That is, the electrons carrying the negative charge are not evenly distributed. This is because the larger and more positively charged oxygen atom exerts a stronger pull on these electrons. The electrons, therefore, spend more time at the oxygen end of the water molecule. As a result the oxygen end of a water molecule is partially (d-) negative and the hydrogen end partially (d+) positive.

A simple experiment that highlights the strength of this attraction can be viewed simply by adding a few drops of water onto oven dry soil. The water molecules are immediately adsorbed, spreading over soil to form a thin film several molecules thick. This is called adhesion water. The extent of adhesion water is though to be about 10 molecules thick and it is not available for plant uptake.
Secondly, water molecules are attracted to each other through hydrogen (H) bonding. This type of bonding is called cohesion and causes water molecules to attach themselves to the adhesion water. Water retained in soil because of cohesive forces is called cohesion water. This water is slightly more mobile and is generally available to plants. The molecules however are not as well orientated as the adhesion water.
Combined the adhesion/cohesion forces cause water films of considerable thickness to be held on soil particle surfaces. Because forces holding water are surface-attractive forces, the more surface area a soil has the greater is the amount of water adsorbed. For practical purposes this means clayier or organic soil can hold much more water than sandy soil.
The remaining soil water is held in aeration pores and is usually removed by drainage because of the force of gravity. It is not normally available to plants as it moves out of the profile within two days of rainfall or irrigation.
Exchangeable cations, such as Calcium (Ca2+), Magnesium (Mg2+), Potassium (K+) and Sodium (Na+), which are common in soil solution and on charged surfaces of clay and humus, are also attracted to the oxygen end of the water molecule (i.e. more negative end). Because of this cations can be removed from the solid matrix of soil particles and into the soil solution.
In the soil solution, the cations are surrounded by a shell of orientated water molecules. This is the normal state of cations in soil solution. They are said to be hydrated. The number of water molecules associated with a cation (i.e. hydrated radius) is a function of the ionic charge and the size of the cation.
| Radius | Unit | Na+ | K+ | Mg2+ | Ca2+ | Al2+ |
| Non-hydrated | nm | 0.095 | 0.133 | 0.066 | 0.099 | 0.050 |
| Hydrated | nm | 0.360 | 0.330 | 0.430 | 0.410 | 0.480 |